H5N1 2.3.4.4b - It's not Just the Dairy Cows and Poultry
And pigs are not the only reassortant risk! Mind the cats!
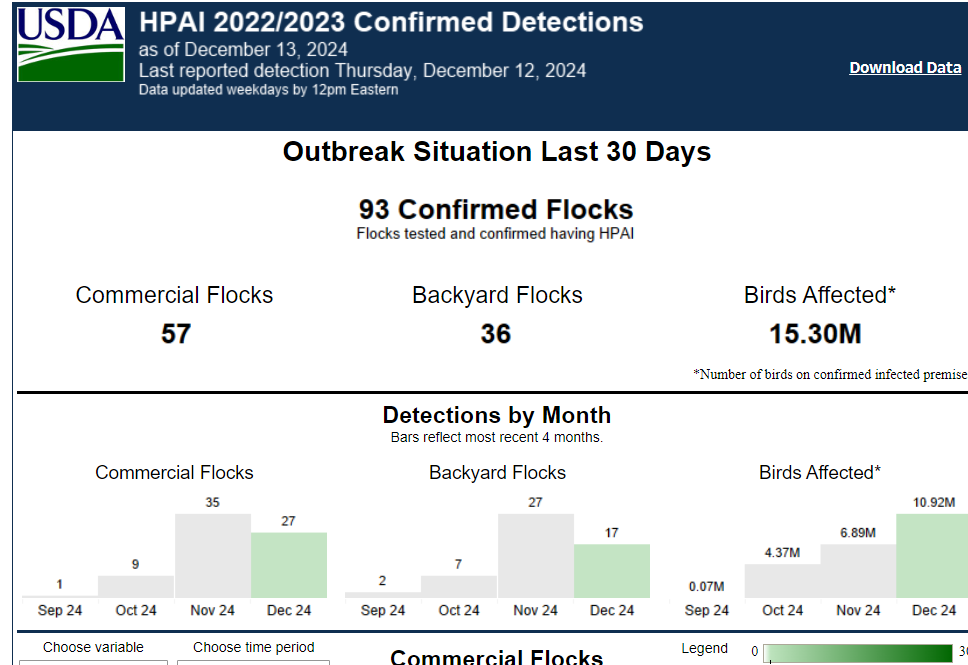
It’s been another depressing week for California’s dairy producers as over 50% of the herds have now been infected with H5N1 (630 cases as of Friday). Its poultry producers are faring no better, with near daily reports of new flocks infected and depopulated. The only difference for poultry producers is that California has been joined by multiple other states in suffering H5N1 avian influenza losses. Here is a screen shot of USDA HPAI Confirmed detections in the last 30 days as of 2:30 PM on December 13th:
Although not divided by viral genotype, most of the California flock outbreaks are likely due to B3.13 spillovers from dairy herd infections, while most of the poultry flock outbreaks in other states are likely due to D1.X genotypes. We still lack full transparency from all stakeholders on this basic information. Regardless, 93 confirmed flock outbreaks in 30 days affecting 15.3 million birds must be some sort of record. According to poultry industry sources, HPAI U.S. layer hen losses for 2024 now exceed 10% of total production. The most endangered farm animal in the United States today may be the laying hen!
It’s now apparent that this fall’s migrating bird population is heavily infected with highly transmissible strains of H5N1 2.3.4.4b D1.2, widely infecting domestic poultry and wild birds and mammals as they move southward this fall. I even saw an anecdotal report of snow geese “falling from the sky”.
Here is a truly sad report from Maricopa County Arizona: Five animals dead after testing positive for bird flu at Wildlife World Zoo:
Zoo officials confirm to ABC15 that five animals have died, including a cheetah, a mountain lion, a swamphen, an Andean goose, and a kookaburra (the latter 3 are birds). A white tiger tested positive for the virus but is expected to make a full recovery.
Viral genotype was not disclosed; however, D1.1 has been diagnosed in poultry in Arizona with no B3.13 in dairy cattle reported. Source of infection at the zoo remains under investigation, with initial wildlife contamination likely. Officials have taken measures to protect and monitor workers for any evidence of H5N1 zoonotic spread.
One of my readers wrote me suggesting that my recent comments regarding threats of H5 viral reassortment in the swine population placed undue emphasis on that species versus risks for the same phenomenon occurring in other species. He has a valid concern, and several publications this week underscore that point. First, a New York Times article reviewed the disasters already playing out in multiple wildlife populations affected by H5N1 2.3.4.4b:
For Wild Animals, the Bird Flu Disaster Is Already Here-NYT
Mammalian scavengers of H5N1 avian mortalities are often H5N1 victims themselves as has been documented with HPAI mammalian wildlife mortalities by USDA: HPAI Detections in Mammals. Along with scavengers, this list also includes cats, mice, and other peridomestic species on or near poultry and dairy premises likely infected by local area viral spread or milk transmission on highly infected sites.
Cats-Perhaps in a Risk Class of Their Own?
Domestic cats have become an area of research interest due to their high mortality from both direct and indirect epidemiological H5N1 exposure as well as zoonotic risks posed by their intimate living relationships with human caretakers. A paper came out late last week on feline H5N1 isolates from Texas, New Mexico and Minnesota: Distribution of lesions and detection of influenza A(H5N1) virus, clade 2.3.4.4b, in ante- and postmortem samples from naturally infected domestic cats on U.S. dairy farms. The abstract drew the following conclusions for guiding diagnostic sampling collection and testing:
In the antemortem samples, the virus was detected by PCR in the oropharyngeal swabs (34.1-36.1), whole-blood samples (30.8-36.6), and one serum sample (31.7). Seroconversion was detected in one cat. Our results support histologic evaluation of brain, lung, eyes, and heart, and PCR testing of brain and lung for postmortem diagnosis, and show that oropharyngeal swabs, urine, serum, and whole blood are suitable samples for antemortem detection of IAV infection in clinically affected cats.
Although the complete paper is currently behind a paywall, I was able to review a full copy and noted an interesting finding regarding ante mortem samples from 2 indoor cats kept strictly inside the home on an infected dairy farm in Minnesota. Both cats were diagnosed via ante mortem sampling with H5N1 2.3.4.4b virus infection, with one of the two reportedly ultimately surviving. Neither cat had direct contact with outdoor animals or milk from the dairy farm. The paper states:
Our findings raise the concern for transmission of H5N1 virus to cats, not only by ingestion of infected material, such as milk or dead birds, but also by contact with fomites or infected material transmitted via the cats’ human handlers, such as owners, keepers, or caregivers. The infection status of the cats’ owners in case 3 is unknown. Overall, a deeper understanding of viral transmission among a higher number of animals and a variety of hosts, from an epidemiologic point of view, is needed. Additional studies investigating the occurrence of horizontal transmission, including contact with body secretions (e.g., saliva, urine), are also warranted
A second study was also released this week regarding sequencing of H5N1 2.3.4.4b B3.13 isolates from deceased household cats from South Dakota:
Full article: Marked Neurotropism and Potential Adaptation of H5N1 Clade 2.3.4.4.b Virus in Naturally Infected Domestic Cats Here are highlights from that text:
In a rural residential area in South Dakota (SD), ten outdoor-housed cats aged 6 months to 4 years were found deceased. Most of these cats were domesticated and regarded as family pets. Clinical signs included anorexia, lethargy, and potential neurologic deficits. Two deceased cats, aged six and eighteen months, were submitted to the North Dakota State University Veterinary Diagnostic Laboratory (NDSU VDL) for postmortem examinations. Along with positive Streptococcus canis respiratory cultures, Polymerase Chain Reaction (PCR) results revealed the presence of influenza A virus (IAV) in the brain and lungs. The Cycle threshold (Ct) values for the IAV matrix gene in the brain were 20.1 and 18.1, and in the lungs were 35.93 and 31.93 for cats 1 and 2, respectively, indicating much higher viral load in the brains of both cats. Samples were submitted to the National Veterinary Services Laboratory (NVSL), Ames, Iowa, and further confirmed to be H5N1 clade 2.3.4.4b by PCR and whole genome sequencing.
A phylogenetic tree of the HA gene from the two cat H5N1 clade 2.3.4.4b sequences was constructed … The resulting maximum likelihood phylogeny tree showed that the HA gene from the South Dakota HPAIV H5N1 clade 2.3.4.4b cat sequences were closest to the H5N1 clade 2.3.4.4b sequences from dairy cattle samples originated from South Dakota and Kansas…
The consensus sequence from the alignment was used as a benchmark to detect the occurrence of mutations in the eight proteins. Two unique mutations were found in the HA (T143A) and neuraminidase (NA) (N71S) proteins. One of the cats H5N1 sequences (GISAID ID # EPI_ISL_19196362) had a mutation in the polymerase acidic (PA) protein (F314L), whereas L342Q mutation was detected in the sequence originating from the other cat (GISAID # EPI_ISL_19196363). There was no evidence of mutations in other proteins….
We further conducted immunohistochemistry on the tissues, targeting the IAV nucleoprotein, which revealed intense staining in a higher proportion of cells in various parts of the brain and intestine. Noticeably, the cerebellum and the hippocampus showed an abundance of nucleoprotein compared to the lungs (Fig. 3, I-L), indicating a higher viral load in brain tissues, as confirmed by the PCR assay…
Our study provides a significant new insight into the neurotropism of the H5N1 clade 2.3.4.4b virus in naturally infected domestic cats. There is a notable shift in the neurotropism of HPAI H5N1 viruses, particularly with the emergence of clade 2.3.4.4b in cats and wild carnivores like foxes.
The co-expression of avian and mammalian SA receptors in cats identified in this study, combined with their potential exposure to various influenza viruses, poses a significant risk for genetic reassortment of different influenza strains, leading to the emergence of novel viral variants.
These 2 articles and other research reviewed certainly raise concerns regarding zoonotic risks for H5N1 2.3.4.4b in cats, especially given their close proximity to people and other hosts such as mice, birds, and fomites like raw milk (link)…
Yes, it’s hard to believe that people are (were, I hope) feeding raw milk to their pets and children. It’s ironic that repeated warnings to people against personally consuming raw milk would be ignored without apparent immediate severe consequences, only to prove lethal to their pet cats… The bottom line is that companion animal veterinarians across the United States and Canada this winter should keep influenza rapid test kits handy for cats (and other companion animals) with respiratory and/or neurological signs, including sudden deaths. Attempted isolation and sequencing at NAHLN labs should be encouraged and ideally should be reimbursed by public health partners for case-compatible submissions. Companion animal owner awareness of zoonotic risks of H5N1 influenza will also be very important.
Once again, companion animal one health surveillance is a regulatory area where no one is leading or supporting even simple organized surveillance processes. This was a big shortcoming with the SARS-CoV-2 pandemic while I was still employed with APHIS, and we’ve made little progress to improve that situation with H5N1! We need these cats diagnosed with any isolates sequenced, especially in areas where H5N1 is ravaging poultry and dairy operations, accompanied by high area viral loads to challenge feline populations in those vicinities.
Let’s not ignore our equine friends…Early Release - Evidence of Influenza A(H5N1) Spillover Infections in Horses, Mongolia - Volume 31, Number 1—January 2025 - Emerging Infectious Diseases journal - CDC. While we have no evidence of clinical H5N1 in horses to date, this manuscript provides good evidence that equids can be infected by H5, albeit at apparently very low levels in this study. Given that horses are endemically infected with H3N8 and H7N7, potential for reassortment cannot be ruled out. Further serological and possibly PCR-based studies would be useful in areas where horses are exposed to high levels of virus from cattle and poultry outbreaks.
Oh, and there are still the pigs (Link)…
Finally, what about goats, sheep, alpacas, farmed elk and deer, AND beef and feedlot cattle? What about non-lactating subpopulations on dairy farms? What percentage of those animals are seroconverting and possibly carrying virus? Is anyone ready to conduct and report the results of strategic sero-surveillance and/or PCR screening of high-risk populations? Has anyone checked retail goat milk and cheese via PCR for H5N1? Can we expect to bring this virus under control and eliminate it in dairy cattle if we have no data on whether it exists or persists in untested populations? Testing bulk milk is a great (late) start, but we need to cover all the bases to assure that lasting success is achievable.
Finally, we have a new “flavor of the month”, H5N1 2.3.4.4b D1.X(1.1-1.2). How does this strain affect mammals? Are dairy cattle susceptible? Could infected poultry flocks “back-infect neighboring livestock operations? Are we conducting precautionary surveillance in neighboring livestock operations adjoining infected poultry flocks to assure we have no subclinical infections with the latest H5N1 2.3.4.4b strain? We already know pigs can be infected (Oregon). If anything, D1.1-2 seems to be more adapted to humans than B3.13; however, that is strictly observational at this early point. We currently await more information regarding a hospitalized patient in Louisiana with H5N1 infection, reportedly with avian exposure of an uncertain nature.
Precautionary surveillance for H5N1 in non-poultry livestock species is not “required”, and animals lacking clear cut clinical signs do not require reporting for examination and molecular screening. Existing animal health rules and regulations allow professional and regulatory “off ramps” from proactive testing responses. But we’re much better than that diagnostically 25 years into the 21st century! We will continue to be at the mercy of this virus and its cousins until we overcome our reluctance to actually use the tools we already have to understand this virus’ movements and pathways, rather than falling back on existing but convenient unmonitored animal movement patterns that spread misery to all.
Let’s strive to make this winter the last one where the egg supply is tanked by HPAI and do our part to make 2025 a year we can continue to travel freely without concern for pandemic influenza threats.
John



Avian flu virus H5N1: No proof for existence, pathogenicity, or pandemic potential; non-“H5N1” causation omitted
https://pmc.ncbi.nlm.nih.gov/articles/PMC7173052/