Molecular Characterization of the Nevada H5N1 2.3.4.4b D1.1 Dairy Cattle Spillover
Prompt sequence releases allow molecular clock estimates for single spillover date and lateral spread to aid in early review of NMTS
A world-wide group of 21 evolutionary molecular experts led by Dr. Michael Worobey of the Department of Ecology and Evolutionary Biology, University of Arizona last night published the following analysis as reported by Mary Wilson on Flutrackers:
Rather than comments from me, I’m simply reproducing the Abstract and portions of the Discussion for your brief review. For a full analysis, please review the entire dispatch.
Abstract
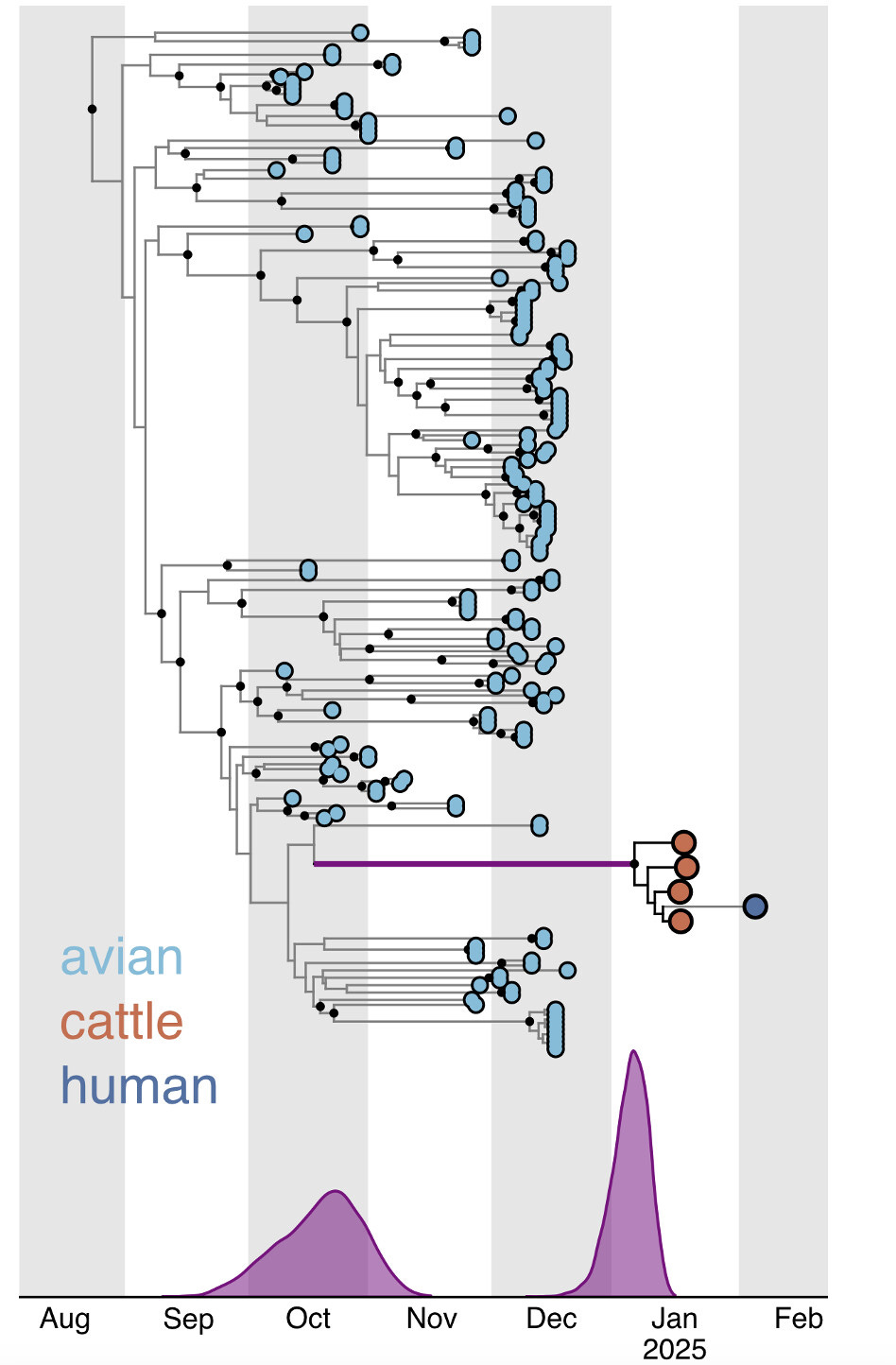
On January 31st, 2025, the United States Department of Agriculture’s (USDA) National Veterinary Services Laboratories identified a new genotype of highly pathogenic avian influenza virus in dairy cattle in Churchill County, Nevada, the second known introduction of clade 2.3.4.4b H5N1 into cattle. Here, we estimate when this virus jumped from the avian reservoir into dairy cattle, using raw sequence reads from four D1.1 bovine H5N1 influenza cases. These data were shared by Animal and Plant Health Inspection Service/USDA on Friday, 7 February 2025. We also characterize mutations in the cattle D1.1 virus sequences and provide a list and brief discussion of mutations that may be of interest or concern. We find that the virus jumped from birds into cattle between late October 2024 and December or early January. Tentative approximations suggest the jump may have happened around the first week of December. This suggests that the origin of this cattle outbreak occurred more than a month before the first quarantines were imposed on two affected farms on January 24th, which had been instituted after the sampling of a local dairy processing plant’s milk silos (January 6th/7th), the testing of these samples (January 10th), and follow-up sample collection (January 17th) and highly pathogenic avian influenza (HPAI) testing (January 24th) at twelve individual farms supplying the silos. Since then, at least four additional infected herds in the area have been identified. Hence, while the discovery of this outbreak illustrates the impressive utility of the National Silo Monitoring Program in detecting outbreaks, our findings suggest that for this program to be most effective in outbreak control, immediate quarantine of all possibly-contributing herds to influenza virus-positive silos might be necessary. Considering the currently widespread nature of H5N1 in the United States, frequent on-site testing, including of individual herds, may be necessary for timely and maximally effective control measures for bovine H5N1 outbreaks.
Discussion
Our findings suggest that the D1.1 outbreak in Nevada was caused by a single introduction of the D1.1 genotype viruses into cattle. However, the four available cattle D1.1 virus genomes appear to come from a single herd, and we do not yet know the extent of this outbreak or the amount of viral diversity currently among the cattle…
The presence of PB2 D701N (nucleotide substitution G2101A; Figure 8) in all four cattle D1.1 genomes (28) suggests a period of evolution within cattle on the stem branch of the four cattle D1.1 viruses (i.e., the purple branch in Figures 2, 3 and 6). This is consistent with the molecular clock analysis that also suggests a period of evolution within cattle, along the stem branch for that clade in our phylogenetic analyses (Figure 7C). …
These findings demonstrate the utility of the National Milk Testing Strategy (NMTS) for identifying novel introductions of H5N1 into dairy cattle. The NMTS was rolled out by APHIS/USDA in December 2024 to provide a more systematic means of testing milk nationally to monitor the outbreak’s spread and identify infected herds. As of February 16, 2025, 42 US states are enrolled in NMTS and conducting active surveillance (1). This detection of D1.1 in milk silo samples from Nevada demonstrates the importance of a systematic, ongoing national milk surveillance approach for early detection, as this second spillover of H5N1 into cattle might not have been identified otherwise.
On January 24th, 2025, the Nevada State Department of Agriculture announced the first quarantines of Churchill County dairy premises (29). (The December 6th, 2024 announcement of quarantines of dairy premises in Nye County, some 250 miles from Churchill County (30), involved a separate (B3.13) outbreak unrelated to the current D1.1 outbreak.) Thus, the time between initial silo sample collection and the first quarantine control measures was 18 days. Our findings are consistent with the quarantines occurring long after spillover, perhaps a month or more.
Encouragingly, the Nevada State Department of Agriculture has communicated that “there have been no movements of dairy cattle within dairies in Nevada, nor any new animals introduced to any dairies within the affected area reported within 30 days prior to the first silo detection. The only movement of cattle out of the affected area are cull cows that have moved directly to slaughter.”
The National Silo Monitoring Program provides a powerful, accessible, and convenient means to detect influenza virus in dairy cattle herds across the country. But it is critical that we close the gaps between outbreak initiation and first detection, and first detection and control measures like quarantines being put into place. The ability to perform the genomic epidemiological analyses, provided by the release of sequencing data from these d1.1-infected cattle by APHIS, and reveals that it may have been more than a month from spillover to quarantines being put into place on the first two herds confirmed to have HPAI in the Northern Nevada outbreak. And as of February 21, 2025 there are now 7 herds confirmed to be infected in this outbreak (31). This may therefore be a case of closing the barn door after the cow has bolted.
For both the National Silo Monitoring Program and the on-farm bulk milk testing programs within the National Milk testing Strategy, we propose the following ideas to shorten the gap between new HPAI introductions to cattle herds and control measure being initiated:
● Immediately place quarantines on all herds that may have contributed milk to a silo that tests positive, then relax quarantines if follow-up testing of samples from individual herds are influenza virus-negative.
● To avoid unnecessary disruptions that the above point would cause, archive, on a rolling basis, small volumes (e.g. 1 L) of milk from each shipment from each farm that is delivered to each silos at processing facilities so that samples from individual premises/herds are available immediately for follow-up testing should a silo test positive.
● For silos, whose large volume might dilute virus present in the milk of a single herd or even a single cow, concentrate virus from a large volume of milk for each test to reduce false-negative influenza A virus PCR results caused by dilution by uninfected milk of infected milk, which will initially rare.
● Develop and employ inexpensive, field-deployable, sensitive rapid tests to avoid delays caused by shipping samples off site and waiting for lab test results, and to allow more frequent testing.
● Where possible, test milk from individual herds to prevent delays caused by having to collect new samples from multiple farms that may have contributed to a positive silo test.
A major driver of the extensive spread of the B3.13 virus through the cattle population was the inability to ascertain the full scope and scale of the outbreak and institute quarantine and animal movement restrictions early on, before the virus transmitted onward to additional herds. The National Milk Testing Strategy and continued testing (and surveillance of wild and domestic birds) are therefore essential to controlling the current cattle epizootic. If the D1.1 lineage has spilled over into a part of the US dairy cattle network previously untouched by, or at least less affected by, the spread of B3.13, then the D1.1 lineage has the potential to result in a large outbreak, like B3.13. The D1.1 genotype is now highly prevalent not only in the US, but also in Canada (e.g., British Columbia), so it is reasonable to anticipate that additional introductions of this genotype to cattle might occur, or might already have occurred, not only in the US but also Canada. Our results highlight the critical importance of surveillance of wild animal monitoring viral spillover into high density populations of agricultural animals more generally which can serve to rapidly amplify and spread pathogens heightening risk of spillover into humans.
John




PubMed was down for a while. No idea how many political changes might have happened during the hiatus, so just mentioning that there is an untainted European alternative.
Europe’s alternative for PubMed for human and veterinary refs
https://europepmc.org/
In ACS Sensors:
ARTICLEFebruary 21, 2025
Capacitive Biosensor for Rapid Detection of Avian (H5N1) Influenza and E. coli in Aerosols