A Week of Papers Published and California H5 Dialogue Amidst More Infected Herds
A picture slowly clarifies that many would prefer to ignore - H5N1 is a systemic infection in cattle, endemic in many affected herds, and soon across the cattle industry
H5N1 is making history every day in the U.S. dairy and poultry industries and is likely a risk in other livestock groups we are only vaguely aware of yet. Whether this virus ever emerges as a full-blown human pandemic, it has already caused billions of dollars of losses across the world in livestock production and even greater damage to our wildlife and marine ecosystems. Some of those costs may not be apparent for some time and are not as easily measured.
Nature Review published a summary authored by a set of highly prestigious authors: The global H5N1 influenza panzootic in mammals | Nature. Here is a direct link to the pdf of the article:
Nature review preprint-Peabody et al the Global H5N1 Influenza Panzootic in Animals_240916.pdf
While somewhat technical, the article provides excellent background information on viral changes already made and additional changes that may be required for H5N1 to adapt for further mammalian spread. I personally found the section related to potential infection in swine quite troubling. I will devote an entire column in the near future to the H5N1 swine risk - as a teaser, remember that California has extensive feral swine populations in the hills near the valley-based dairy farms, as well as a fairly robust outdoor exhibition and small producer pig industry.
Another preprint publication reached final clearance this week: H5N1 clade 2.3.4.4b dynamics in experimentally infected calves and cows | Nature This is the work by the Loeffler Institute and Kansas State University released earlier in preprint related to intranasal infection of calves with an onward transmission challenge with H5N1 2.3.4.4b B3.13 (Kansas State) and intramammary infusion of lactating cows with that strain plus another European H5N1 2.3.4.4 b strain (Loeffler). The work, results, and conclusions remain unchanged by my reading; however, the authors did make some additional comments in the Discussion that I think are worth noting as epidemiological evidence continues to mount contradicting their central assertion that much of the intra- and inter-herd transmission of H5N1 in dairy cattle occurs via ascending (mechanical) teat/udder transmission.
One section in the discussion states: The possibility for non-lactating cattle to serve as a virus source for onward transmission to adult dairy cows, poultry or mammalian species such as felines, should be considered as we observed nasal shedding of infectious virus for 7 days. The same scenario also exists for environments contaminated with milk from H5N1infected cows. And the final sentence perhaps is most important: Focused efforts to better elucidate transmission pathways and H5N1 ecology in the dairy industry worldwide are critically needed.
In the meantime, the California dairy industry had a rough week, with 43 herds now confirmed to be infected with H5N1, leading to extensive state bulk tank testing of linked and neighboring dairies. I would expect many more herds will be confirmed this week as the state hits the logarithmic section of the epidemic curve for this outbreak. One infected turkey flock has already been reported, and many more poultry outbreaks are almost a given, with the dairy and poultry facilities so closely co-located in the center of the state.
The H5N1 outbreaks in California led to at least 2 sponsored dairy producer webinars I heard of but did not personally listen in on. The first one featured Dr. Jason Lombard of Colorado State University, an excellent dairy veterinarian and former USDA-APHIS-Veterinary Services Epidemiologist. Jason has been invited to on-farm investigations of dairy H5N1 cases in multiple states. I received screenshots of excellent information he provided on the webinar. I won’t share them directly since I didn’t attend virtually but can summarize several important messages extracted from his slides:
18 milk transport trucks were sampled in multiple spots with a total of 126 swabs taken. Only one swab on one truck was positive, indicating milk transport trucks are likely of negligible risk for spreading H5 virus between farms.
Multiple cows were pictured with serous to bloody nasal discharge, indicating a definite upper respiratory component to this disease in lactating cattle.
Eating, rumination, and activity all dropped quickly in affected cows as recorded by activity monitors; average herd rumination and milk production dropped precipitously for 2 weeks, and milk production only returned to 70-75% of pre-infection levels across compiled herd level records in a herd with networked rumen monitors.
As previously reported by Dr. Drew Magstadt of Iowa State University, H5 virus PCR markers appeared in bulk tank samples 15-17 days prior to appearance of clinical signs, indicating longstanding viremia in cows prior to initiation of mastitis. Further tests showed that serological H5 ELISA tests become positive on about day 7 after clinical signs and remained positive as PCR results became negative around day 22 in the herd studied.
On one farm with longer-term bulk tank testing, PCR results became negative around day 40 but dipped into the high 30’s periodically for up to 100 days post infection, indicating some residual herd reinfection. This is in accordance with the indirect evidence for recurrent longer term herd reinfections I extracted from the Colorado bulk tank testing results in a previous blog- Where are We At? It's in the (Bulk) Tank (substack.com).
PCR positive samples were collected from milk, respiratory swabs and urine, with milk positives more common and with lower CT values on a few farms. (These samples were likely taken from cows after the herds were clinically diagnosed, i.e. mastitis had appeared). The presence of virus in respiratory and urine samples once again indicate systemic presence of virus, versus a localized udder infection.
Dr. Jennifer Walker posted commentary on LinkedIn Saturday regarding a second California dairy producers’ webinar dealing with clinical management of sick cows with H5 infections: https://www.linkedin.com/feed/update/urn:li:activity:7245921105599762432/
Dr. Walker is obviously also very frustrated regarding several issues upon which I’ve elaborated previously. Some of the treatment short-cuts advocated in the field are concerning as well, as are cow welfare and employee health issues. Read through her post, including the screenshots of her .pdf for many unvarnished insights into the clinical picture and field management issues with H5 infections in large dairy herds.
I’d also like to reference a slide from a presentation early last week at the Leman Conference by Dr. Bailey Arruda from the National Animal Disease Center (ARS) in Ames referring to ongoing bovine H5 research:
Note that bucket calves fed H5N1 infected milk became ill, showing mild clinical signs with PCR detection of virus shed from the upper respiratory tract. Calves also seroconverted to the infection. This work confirms that calves are susceptible to oral viral inoculation, as well as to intranasal inoculation as previously demonstrated in earlier work.
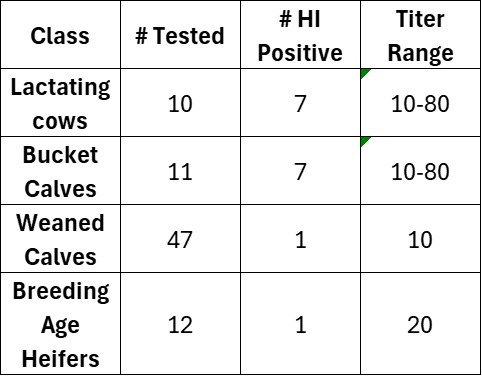
Dr Kay Russo, with RSM Consulting, LLC based in Colorado, conducted limited sero-surveillance of multiple groups of cattle on a quarantined farm, with the following results:
Note that the bucket calves on this quarantined farm also seroconverted via HI titration, either due to active infection or possibly due to absorption of colostral antibodies from ingested milk if born and fed milk post-outbreak. These calves were reportedly fed pasteurized milk from the infected herd; however, there may have been issues with the pasteurization equipment. Some of the bucket calves (pictured) also exhibited striking epidermal erosions on the muzzle, possibly signaling resolving upper respiratory infections. PCR samples from these lesions were negative.
Returning to the HI serology results summarized above, note that 1 weaned calf and 1 breeding age heifer on this farm also seroconverted, indicating low levels of within farm non-contact spread from the lactating herd to other production groups on the farm.
I had a message exchange with a well-connected dairy veterinarian with extensive H1 outbreak experience regarding the entire disease process in acutely ill cows, i.e. what is driving the decreased digestive motility, high fevers, and rapid dehydration? Here is an edited transcript of our conversation:
John: Do all the sick cows have mastitis, or are they sick AHEAD of the clinical mastitis? The reason I ask about mastitis is because of the question whether the mastitis is causing the fever or if there are other factors, like viremia at play - XXX and I were discussing the possibility of neurologic involvement leading to loss of rumen motility, no drinking, etc.
Yeah that's a great question on primary cause of fever. They are seeing more cows with vasculitis leading to cows with nosebleeds.
Same thing can be happening in the udder as well, leading to what looks like a toxic mastitis
Feedback from vets in regions affected is that they are seeing some clinical mastitis but not all
Neuro maybe or perhaps the fact that you have a bunch of leaky vessels all of a sudden all over the body
John: I've been of the opinion that H5 is a viremic infection, with mastitis as a common sequala in lactating cows. People have it backwards claiming that it starts in the udder.
Agree, the mastitis is a clinical symptom - not the primary cause
Here we observed 'digestive' cows prior to seeing any mastitis at all
And cows with tons of nasal discharge, the 'drooling' cows
The collared cows too with rumination information, that was the first indication if you have wearables
John: Also think that milk goes viremic (bulk tank positive) long before the udder is actually infected -i.e. the virus leaks through the blood stream into the milk.
100% this
What part of her body has a massive amount of vasculature and blood flow? Udder
We all remain frustrated that transmission, viral receptor sites, and even pathogenesis in lactating cows remains such a mystery. What acute infectious process leads to the sudden fevers, dehydration, and loss in digestive motility in so many animals in the field, when such conditions can’t be replicated in laboratory direct infection studies?
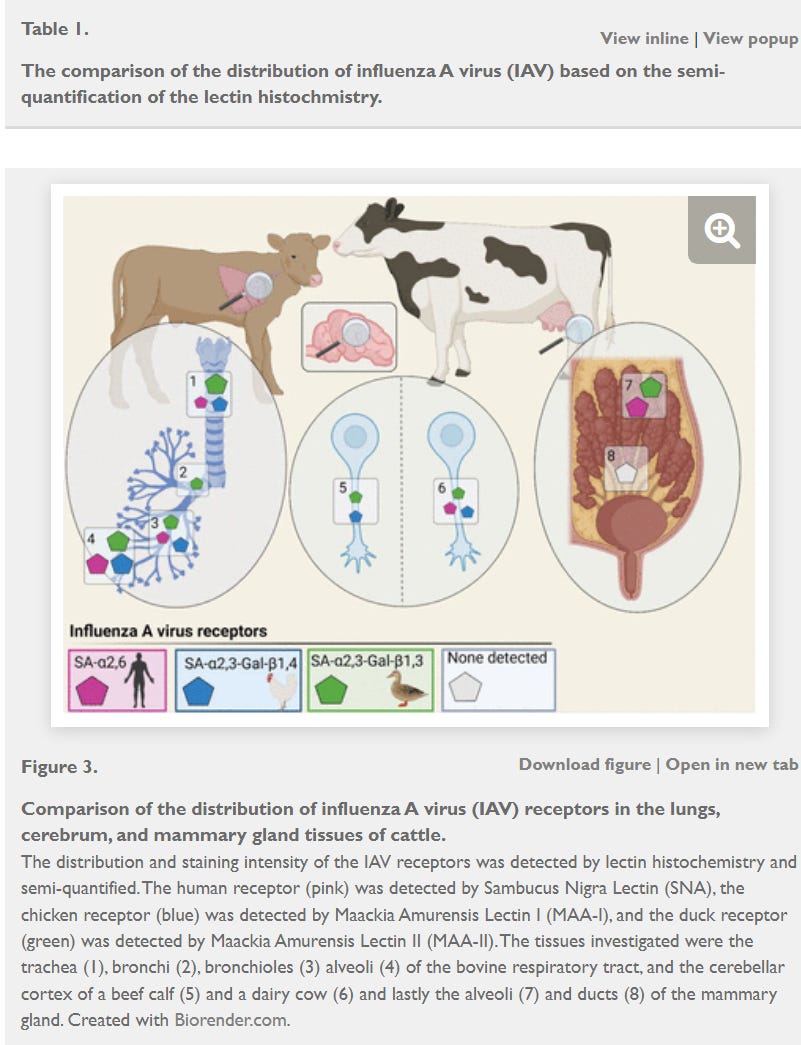
One reason I had speculated on a possible neurological role in this syndrome relates to work published in a preprint by Kristensen et al in bioRxiv back in May:
These graphics nicely illustrate H5 attachment friendly sites within cattle as determined via receptor-specific staining methods in the respiratory tract, udder, and brains of calves and lactating cattle. While the secretory glands of the udder had the most abundant sites, 2,3 attachment sites were also found in the respiratory sites and nervous tissue. Additionally, final release from Loeffler-Kansas State (H5N1 clade 2.3.4.4b dynamics in experimentally infected calves and cows | Nature) found evidence of H5 in brain tissue via PCR in cows infused with both the American and European H5 strains, although no histologic or antigenic microscopic lesions were found. It is at least plausible that some sort of central nervous system depression could be involved with the digestive dysfunction.
Alternatively, the increasing signs of vasculitis noted in many cases are another promising area for further investigation. Diehl et al showed viral presence via in situ hybridization and immunohistochemistry in the following tissues in dairy cattle in their early studies published back in July - Spillover of highly pathogenic avian influenza H5N1 virus to dairy cattle | Nature. Note that blood vessel endothelial cells, cardiomyocytes, and the spleen were all affected by H5 virus:
We need to get back to some of the early findings outside of the udder to review the systemic effects of this virus prior to the final mastitis stages found in a subset of the affected animals. Bottom line, H5N1 attacks herds long before mastitis appears in a subset of cows, triggering the official USDA H5 case definition.
The industry badly needs:
Routine bulk tank testing of high-risk herds to identify infection at its inception, followed by intense monitoring of a subset of newly infected herds at the onset of positive findings; volunteer infected farms are critical for intense epidemiological and clinical monitoring. California now is the epicenter, and UC-Davis as a premier veterinary college needs to step forward to coordinate multi-institutional investigations.
Clinicians need to “live” on a few farms for daily extensive sampling, clinical assessment, and testing of affected cows from the onset of clinical signs, including
full post-mortems and extensive histology on any acutely affected mortalities
All participants should commit to rapid wide-spread public sharing of all findings for replication and verification across multiple herds in multiple localities.
Epidemiologists and environmental engineers should develop well-conceived aerosol, surface, rodent-insect, and fomite sampling and assessment protocols for transmission on and between affected farms - at the time of infection when viral levels are peaking.
Once researchers better understand pathogenesis in sick animals and viral loads at the animals’ head on the farm, determining the exact mode(s) of transmission should become more straight-forward. The virus by definition almost has to enter via the head - eyes, nose or mouth. Where are the receptors and what is the minimal dose? Is co-infection required? Why does H5 spread so rapidly on and between herd mates and farms, but not well at all in BSL-3 labs? Why do some animals get so sick in the field, but experimentally infected animals exhibit minor illnesses? I think we need to start at the farm at this point, then work backwards.
As part of the summary in the Nature Review Preprint, Peabody et al asked the following:
Can H5N1 be eliminated in US dairy cattle? Two features of the H5N1 outbreak in bovine make eradication feasible. First, most transmission appears to occur through a defined pathway via milking machinery instead of the more diffuse respiratory route. Hygiene and biosecurity improvements could potentially break transmission. Second, spillover from wild birds into dairy cattle appears to be rare. If US dairy farmers could manage to eliminate the current H5N1 outbreak through a combination of biosecurity, testing, quarantine, real-time genomic epidemiology, and possibly vaccination and/or culling, the virus may not return from wild birds. However, six months into the outbreak, the proverbial cow may already be out of the barn.
I would argue that ample epidemiological evidence of explosive spread as well as the systemic clinical picture outlined above forecloses on any possibility for eradication from cattle in the near or intermediate term. We need to accept that reality because milk (fomite)-udder transmission described above does not fit the evidence. The disease is: 1) much more systemic in affected animals; 2) more widespread beyond milking lines on affected farms; 3) spreads more rapidly and easily between farms than is realistic with strictly fomite-driven biosecurity lapses; and 4) likely extends beyond dairy farms to other cattle if we are willing to look. It’s not a pretty picture, but these are Stubborn Facts, despite current Stubborn Fallacies.
John